
Electron discovery
It is considered to be the first to introduce the idea of the divisibility of matter into many small and therefore invisible particles, atoms, advanced by the ancient Greek philosopher Leucippus. The doctrine of Leucippus was developed by his student Democritus, who argued that atoms are eternal, immutable and indivisible particles of matter moving in absolutely empty space, and changes in the Universe occur solely due to changes in the bonds between atoms. Similar views dominated in science for more than two thousand years, and until the beginning of the XIX century the existence of atoms remained only a hypothesis.
Confirmation of their existence was made during the establishment of empirical laws of physical and chemical processes, in particular, the work of the Englishman John Dalton, who introduced the concept of atomic weight and was the first to calculate it in relative units.
In 1844, the English surgeon Richard Laming was the first to hypothesize that an atom consists of a nucleus surrounded by charged electrical particles. Laming did not cite any experimental data in support of his theory, and his report to the Royal Scientific Society was ignored for many years.
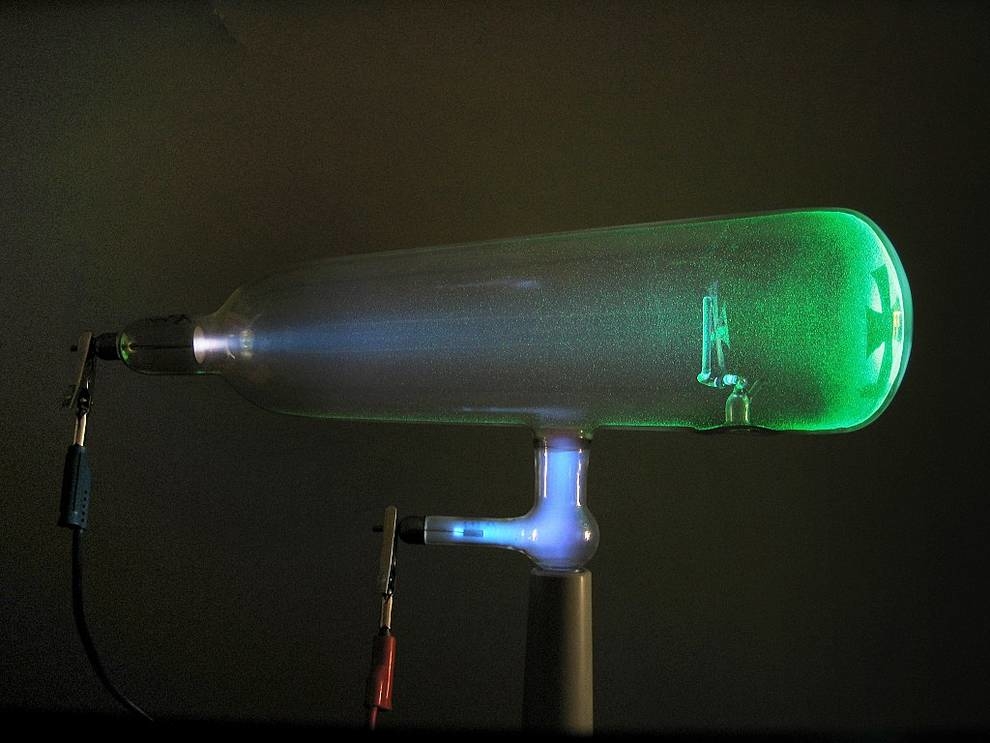
The next step in the study of the atom was the study of the phenomenon discovered in 1869 by the German Johann Hittorf in the study of the conductivity of gas between two electrodes under reduced pressure and low voltage. Unusual luminescence at the cathode (positively charged electrode), depending on the degree of rarefaction of the gas, was subsequently studied by the Englishman Crooks, who established that it moves from the cathode to the anode, it can be controlled by a magnetic field and the “carrier glow” has a negative charge.
The fundamental nature of cathode rays was studied by the physics professor Joseph Thomson, who, along with his colleagues John Townsend and Harold Wilson, conducted a series of experiments at the Cavendish Laboratory of the University of Cambridge. Using an improved “Crookes tube,” supplemented by electric coils and a set of parallel electric capacitor plates, they created a magnetic and electric field inside it.
On April 30, 1897, Joseph Thomson gave a report to the Royal Society of London. He reported on the results of electro-magnetic studies of the emission of cathode tubes, which made it possible to establish the existence of a light subatomic particle with a negative charge, which he called the "corpuscle". This discovery revolutionized the concept of the structure of the material world, and in 1907 Thomson was awarded the Nobel Prize in Physics.
Confirmation of their existence was made during the establishment of empirical laws of physical and chemical processes, in particular, the work of the Englishman John Dalton, who introduced the concept of atomic weight and was the first to calculate it in relative units.
In 1844, the English surgeon Richard Laming was the first to hypothesize that an atom consists of a nucleus surrounded by charged electrical particles. Laming did not cite any experimental data in support of his theory, and his report to the Royal Scientific Society was ignored for many years.

Photo © jnsm.com.ua
The next step in the study of the atom was the study of the phenomenon discovered in 1869 by the German Johann Hittorf in the study of the conductivity of gas between two electrodes under reduced pressure and low voltage. Unusual luminescence at the cathode (positively charged electrode), depending on the degree of rarefaction of the gas, was subsequently studied by the Englishman Crooks, who established that it moves from the cathode to the anode, it can be controlled by a magnetic field and the “carrier glow” has a negative charge.
The fundamental nature of cathode rays was studied by the physics professor Joseph Thomson, who, along with his colleagues John Townsend and Harold Wilson, conducted a series of experiments at the Cavendish Laboratory of the University of Cambridge. Using an improved “Crookes tube,” supplemented by electric coils and a set of parallel electric capacitor plates, they created a magnetic and electric field inside it.
On April 30, 1897, Joseph Thomson gave a report to the Royal Society of London. He reported on the results of electro-magnetic studies of the emission of cathode tubes, which made it possible to establish the existence of a light subatomic particle with a negative charge, which he called the "corpuscle". This discovery revolutionized the concept of the structure of the material world, and in 1907 Thomson was awarded the Nobel Prize in Physics.


